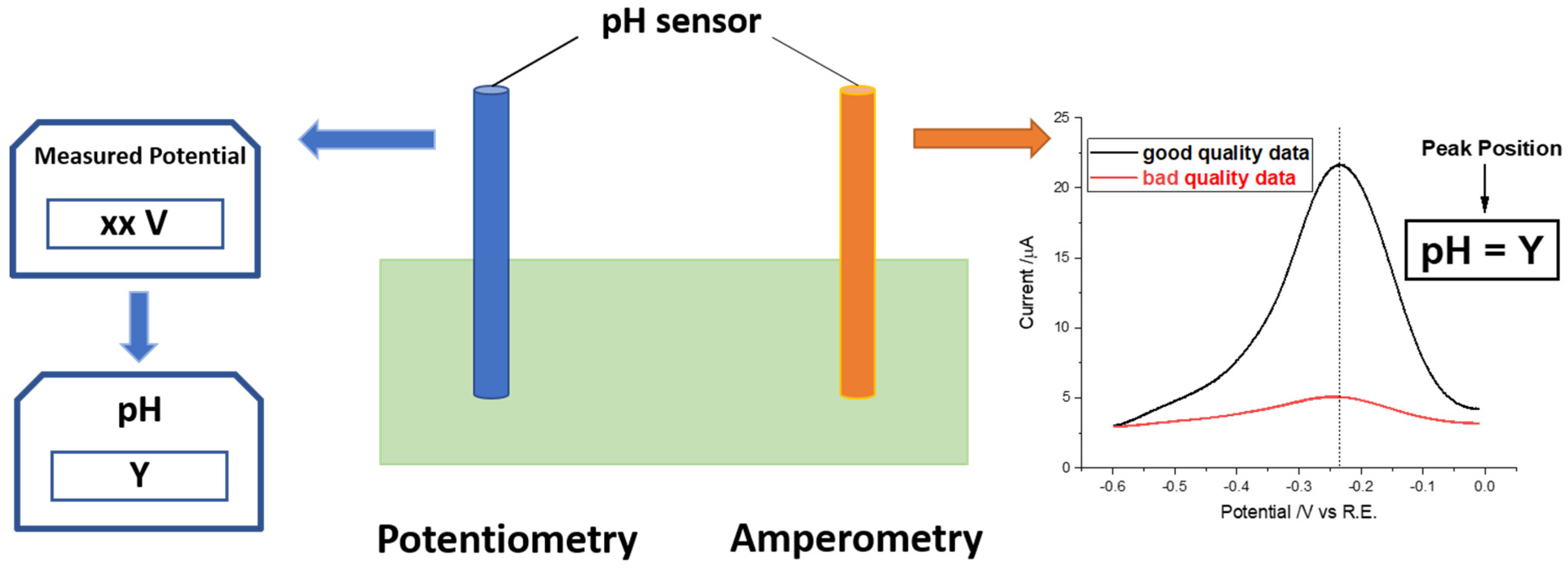
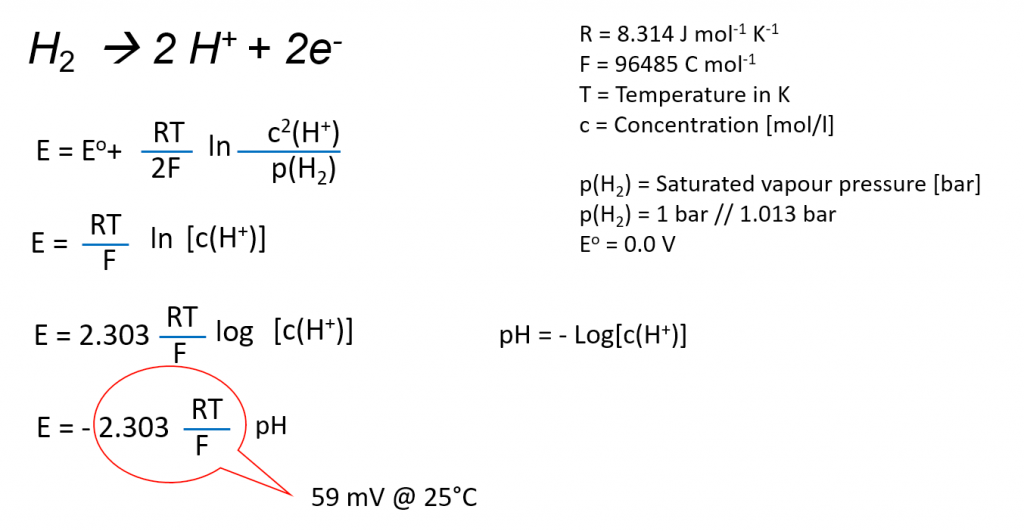
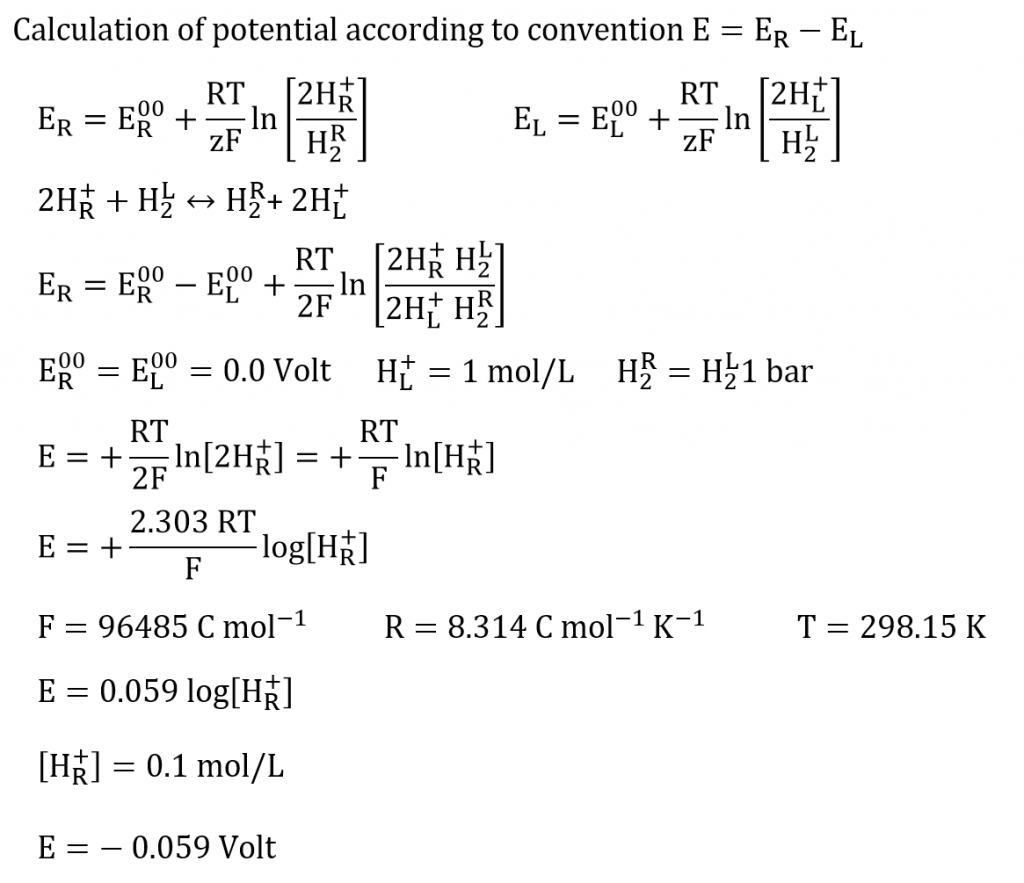
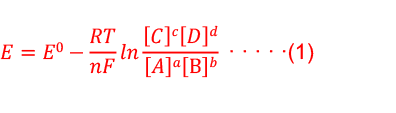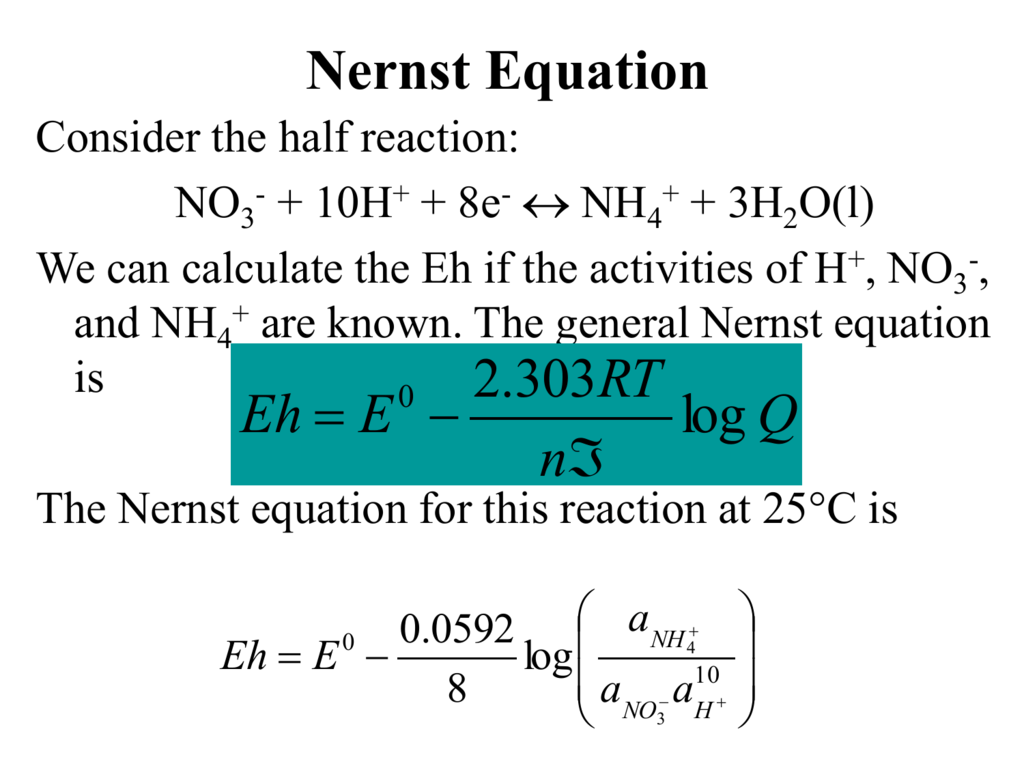A novel sensor based on electropolymerized substituted-phenols for pH detection in unbuffered systems - RSC Advances (RSC Publishing) DOI:10.1039/C5RA22595G
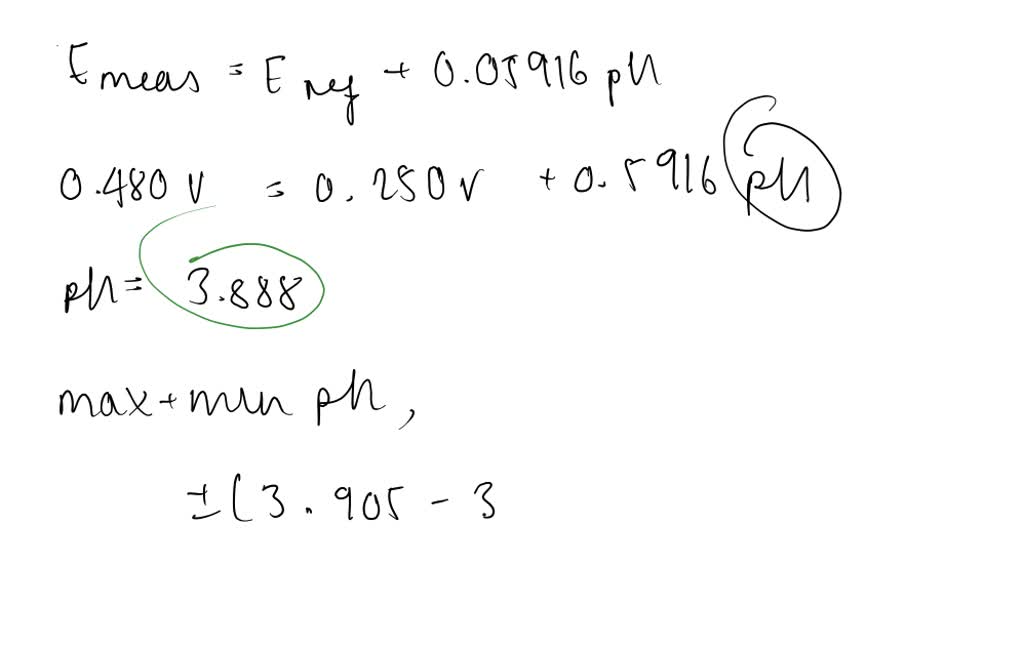
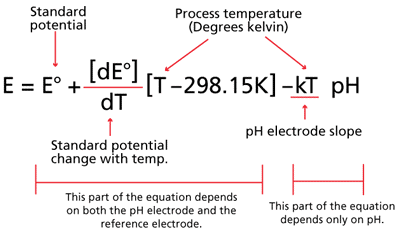
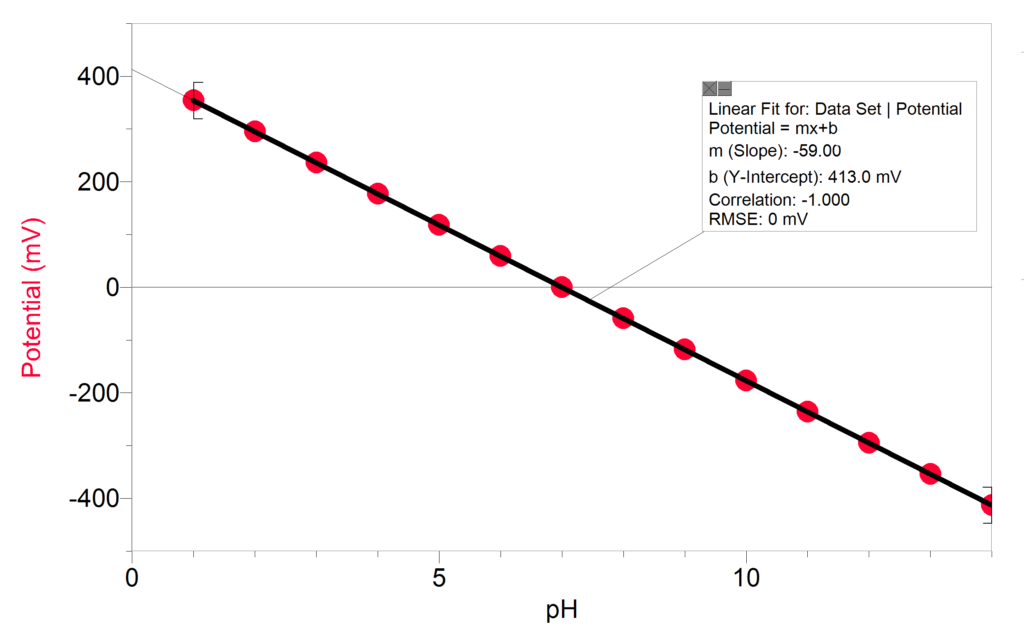
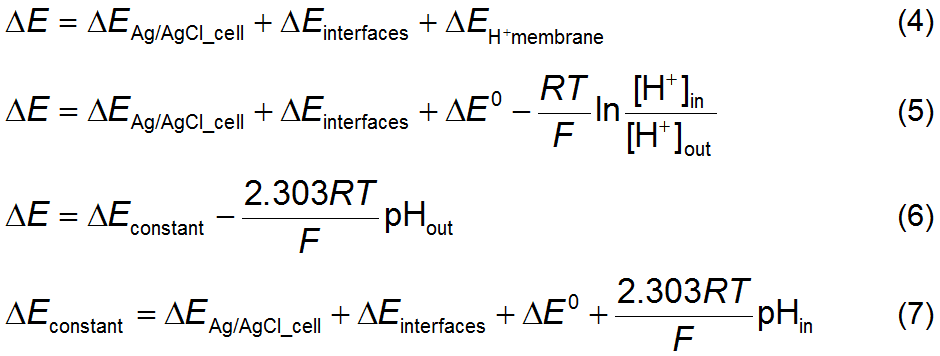
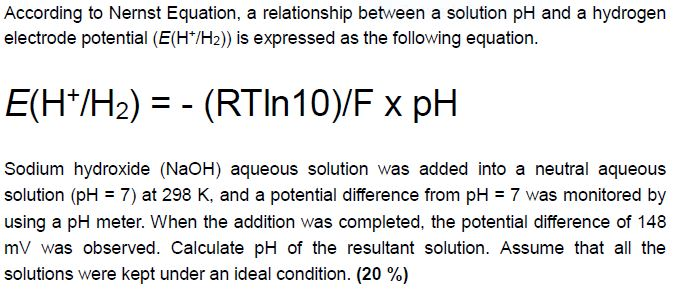
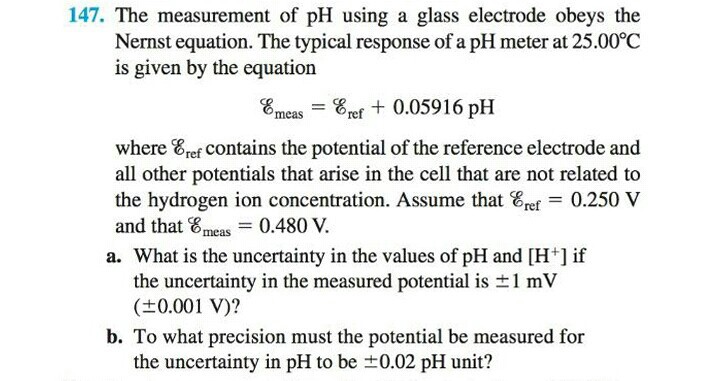
SOLVED:The measurement of pH using a glass electrode obeys the Nernst equation. The typical response of a pH meter at 25.00^∘ C is given by the equation ℰ meas =ℰ ref +0.05916
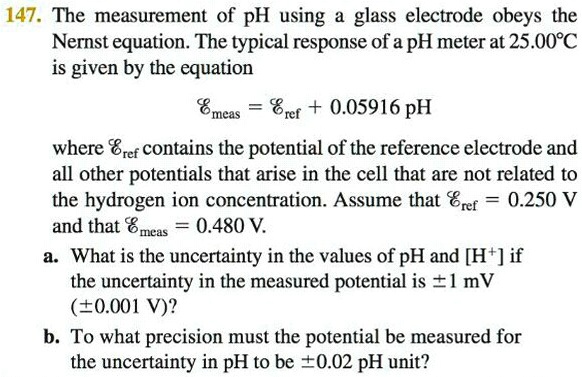
SOLVED: 147. The measurement of pH using glass electrode obeys the Nernst equation. The typical response of a pH meter at 25.00PC is given by the equation 4 meas 9ref + 0.05916
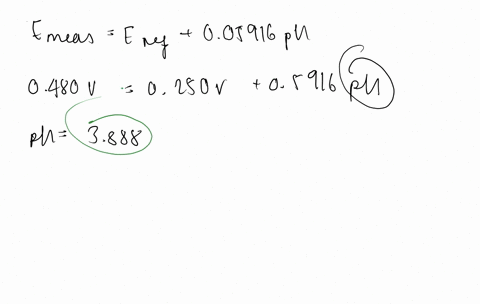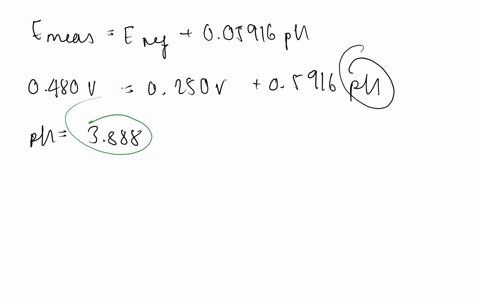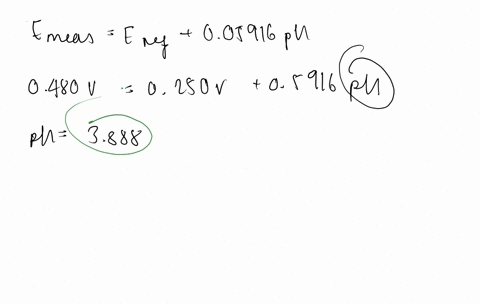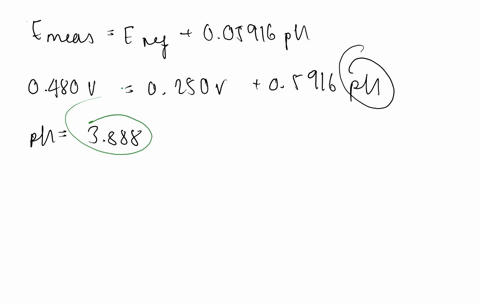
SOLVED:The measurement of pH using a glass electrode obeys the Nernst equation. The typical response of a pH meter at 25.00^∘ C is given by the equation ℰ meas =ℰ ref +0.05916
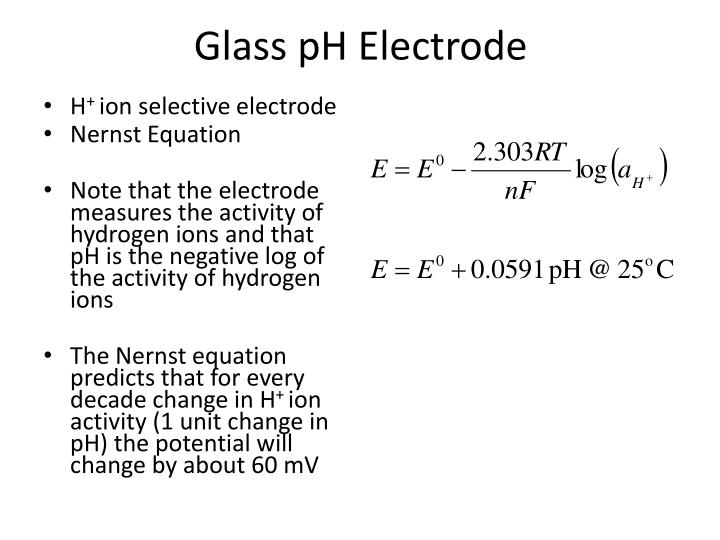
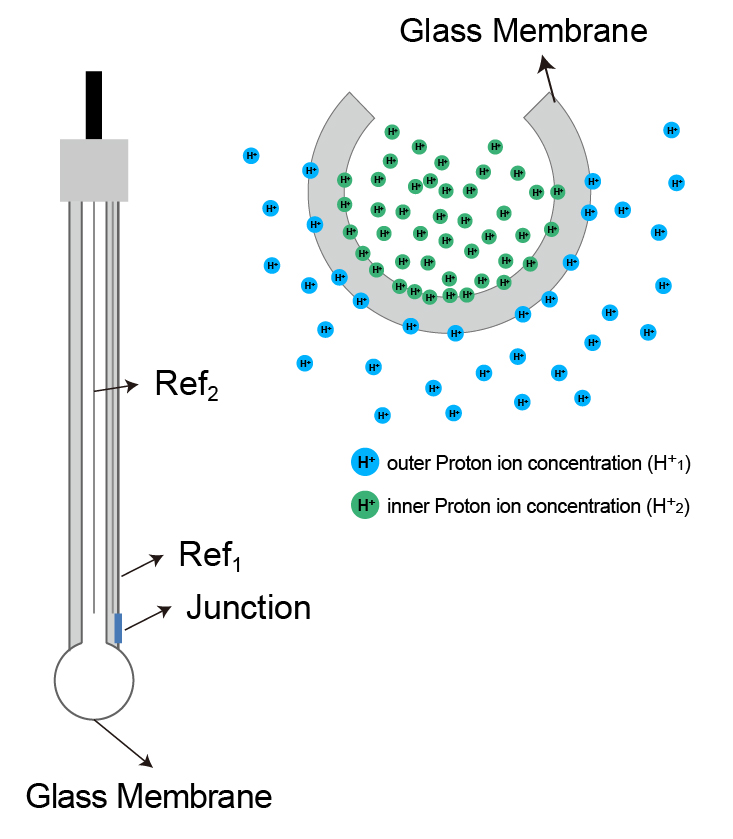
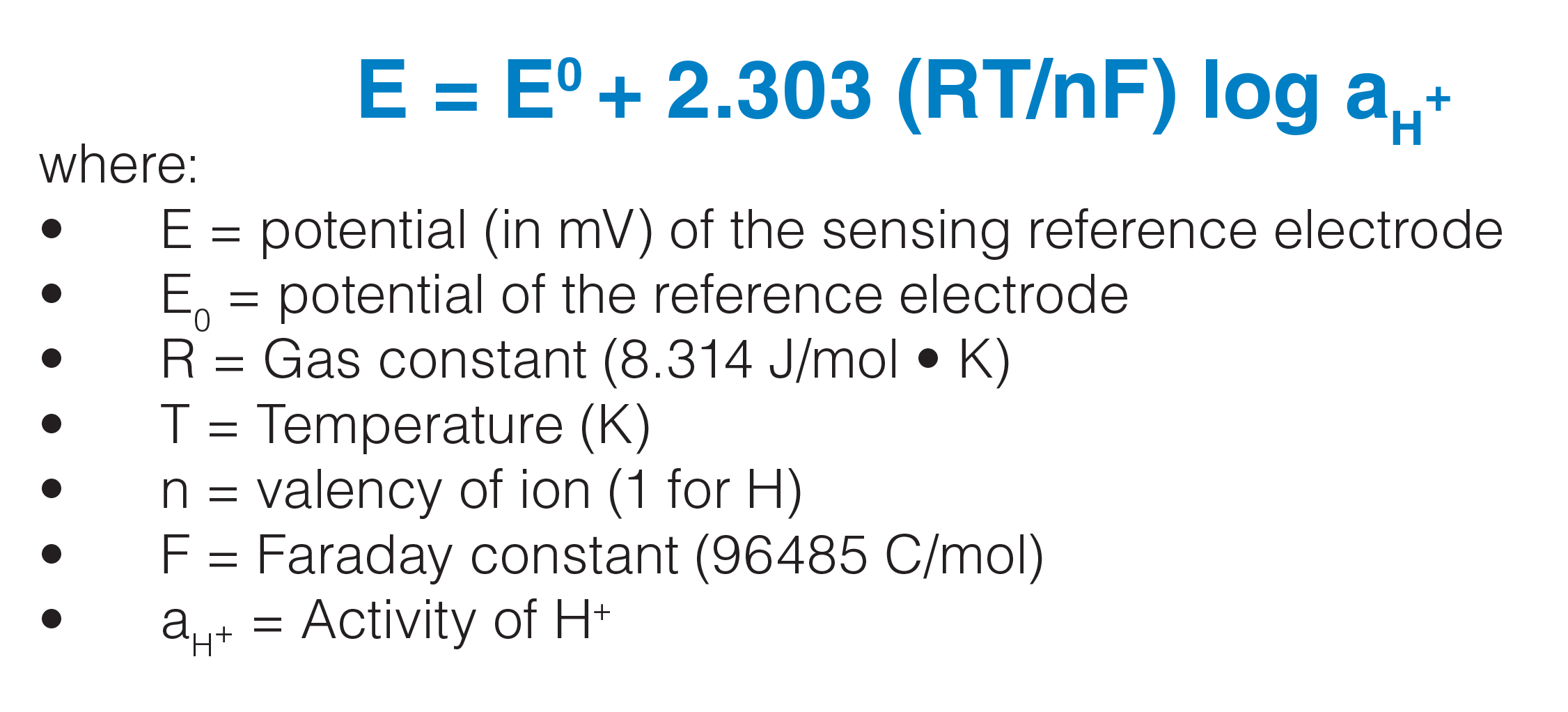
PPT - Care and Maintenance of Electrodes for pH and Voltametric Measurements PowerPoint Presentation - ID:460665